Reliability of the Three Most Common Glucometers in Morocco as Influenced by Blood Content of Dextrose, EDTA, Mannitol, and Urea
Kawtar Layouni1,*, Chaimae Lahlioui, Mohammed Diouri1,*
1 Moulay Ismail University of Meknes, Morocco
* k.layouni@edu.umi.ac.ma; m.diouri@umi.ac.ma
Abstract: This study aims to evaluate the reliability of the glucometers that are commonly used in Morocco, and to assess the effect, on glycemia determination, of blood content of different substances reflecting various health and nutritional conditions. One of four interferences (dextrose, EDTA, mannitol, or urea) was added, at one of four concentrations (0, 100, 200, or 300 mg/dL) to human blood containing one of two levels of glucose. Blood glucose (BG) was assayed in an accredited private analysis laboratory and by glucometers, belonging to three brands. The different interferences, except dextrose, did not affect BG. BG values of the glucometers were 22% higher than those of the lab (p<0.05), but highly correlated with them (r=0.95, p<0.001). The glucometers used in Morocco are precise enough to be used to follow glycemia evolution. However, they should be better calibrated before sale for a better accuracy that allows the exact BG determination.
Keywords: Glucometer, Diabetes, Accuracy, Precision, Self-Monitoring of blood glucose (SMBG), glycemia, interference.
Received: April 05, 2025
Revised: May 25, 2025
Accepted: June 11, 2025
Published: July 25, 2025
Citation: Chouikh S.E., Boualam A., Zamd M., Aittaleb A. Behavioral approach of a morwak ultrafiltration device. Moroccan Journal of Health and Innovation (MJHI) 2025, Vol 1, No 2. https://mjhi-smb.com
Copyright: © 2025 by the authors.
- Introduction
Diabetes is a common chronic disease, causing different complications (Heald et al., 2020). The world prevalence of diabetes reached 9% (463 million adults) in 2019, due to a decreasing mortality among diabetics, because of the improved medical care, and to increasing risk factors (Chan et al., 2020; Magliano et al., 2019).
The approach of “Self-Monitoring of Blood Glucose” (SMBG) has been suggested by researchers for burden reducing and cost-effectiveness improving (Kalatehjary et al., 2008; Lagarde et al., 2006). SMBG is a process of Blood Glucose (BG) checking by the patients themselves, thereby increasing their self-confidence (Bergenstal et al., 2005; Czupryniak et al., 2014). SMBG is commonly applied three times a day (American Diabetes Association, 2016). The awareness of diabetic patients about the advantages of SMBG has risen, so its utilization has rapidly increased in recent years (Gomes et al., 2010). However, the precision and accuracy of SMBG devices are doubtful (Freckmann et al., 2010), making it risky to rely on for a clinical decision (Van den Berghe et al., 2001).
This study aims to evaluate the precision and accuracy of the glucometers that are commonly used in Morocco (OnCall Extra, CareSens, and GlucoLab brands), and to assess the effect, on BG determination, of blood content of different substances (dextrose, EDTA, mannitol, and urea) reflecting various health and nutritional conditions.
2. Material and methods
Approximately 200 mL of blood was collected, from 10 volunteers, in heparin tubes. Half of this amount was centrifuged and frozen (this was the “High” blood). The other half (“Low” blood) was left for 24 hours at room temperature (to allow glycolysis) and then centrifuged and frozen. The two plasma pools were defrosted just before use. The volume of each pool was about 45 mL.
Plasma was distributed over test tubes. The first series of tubes, numbered 1 to 13, contained a constant volume (2.5 mL) of High plasma. The second series (14 to 26) contained the same volume (2.5 mL) of Low plasma. One of four interferences (dextrose, EDTA, mannitol, or urea) was added to each tube at one of four concentrations (0, 100, 200, or 300 mg/dL), and mixed by hand. tubes 1 and 14, considered as controls, did not receive any interference (Concentration 0) and were repeated twice.
Part of the plasma from each tube was transferred to Eppendorf tubes and sent to an accredited private analysis laboratory (Elmaadani) in Meknes, Morocco. The remaining part was assayed for Glycemia, using three different glucometers, belonging to different brands, and different countries, OnCall (USA), CareSens and GlucoLab (South Korea). A set of four plasma samples was also analyzed in another accredited private laboratory (Biougnach, Meknes, Morocco) to compare and verify laboratory results.
All treatments and measurements were performed randomly. The glucometers used in this study were calibrated by the seller to simulate the common behavior of the patients. data were analyzed, using R-software (Core Team, 2024), by ANOVA and pairwise T-test. Kruskal-Wallis nonparametric test was also used when conditions of these procedures were not met.
- Results and Discussion
Results were not lab dependent (p=0.12). The lab values were then considered good bases of comparison for our glucometer results.
The two plasmas (High and Low) were significantly different in glycemia. The duration of 24 h was not sufficient to deplete the blood from glucose. Indeed, The High and the Low controls had a BG of 99 and 45 mg/dL (figure 1), respectively. The collected blood pool (High) was normal with respect to glycemia.
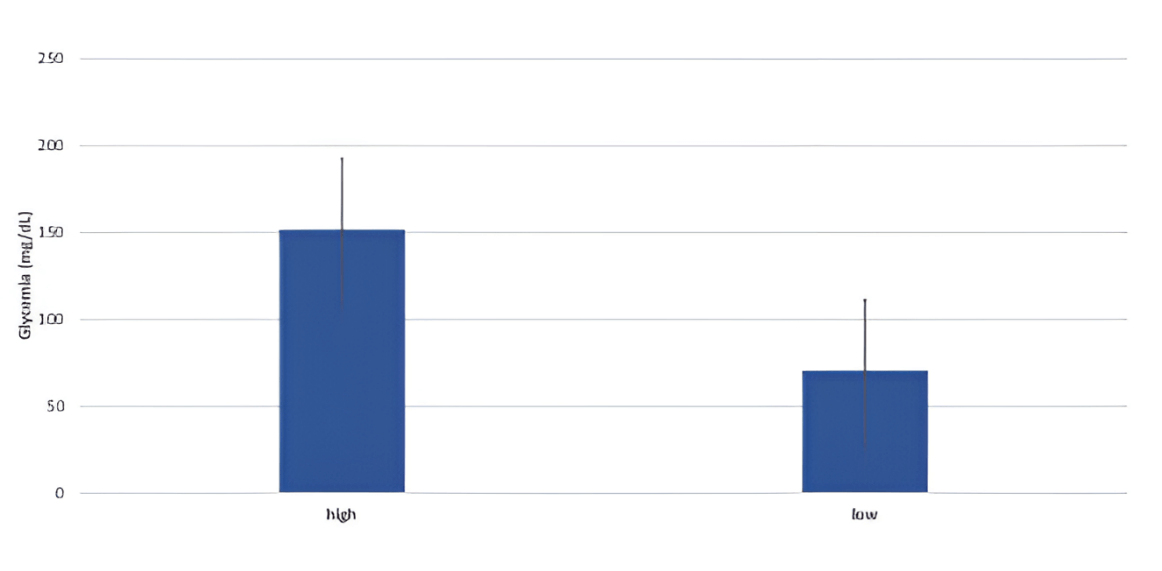
Figure 1: Mean glycemia of the High (frozen after collection) and the Low (left at room temperature for 24h) plasmas. Error bars represent the standard error.
The different interferences, except dextrose, did not affect glycemia (figure 2). This was true for all the concentrations tested. In fact, concentration and glycemia (measured in the lab) were correlated (r=0.95, p<0.001) For plasmas containing dextrose; but not correlated (r=0.003, p>0.05) for plasmas containing one of the other interferences.
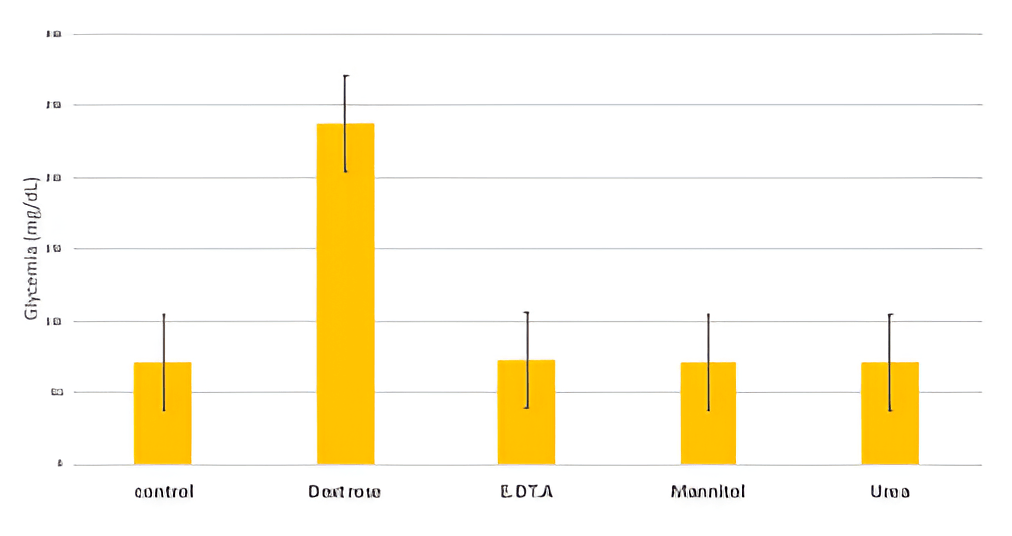
Figure 2: Glycemia of plasmas containing different Interferences. The control plasma does not contain any Interference. Error bars represent the standard error.
The average BG measured by the three glucometers was significantly higher than that of the lab, except for CareSens (figure 3). This difference averaged 22% (15.4 for Low blood and 28.6 for High blood with p<0.05), making the glucometer not reliable for the determination of the exact BG. However, the results of the glucometers were correlated with those of the Lab (r=0.95, p<0.001). These glucometers are then precise but not accurate. Since our study used only one glucometer per brand, we are not allowed to conclude that CareSens is more accurate than the others. The outstanding result of this glucometer may only be due to good calibration.
Although the difference between glucometer and lab readings was significant, it was not affected by the interferences. This makes our study results applicable to different blood compositions.
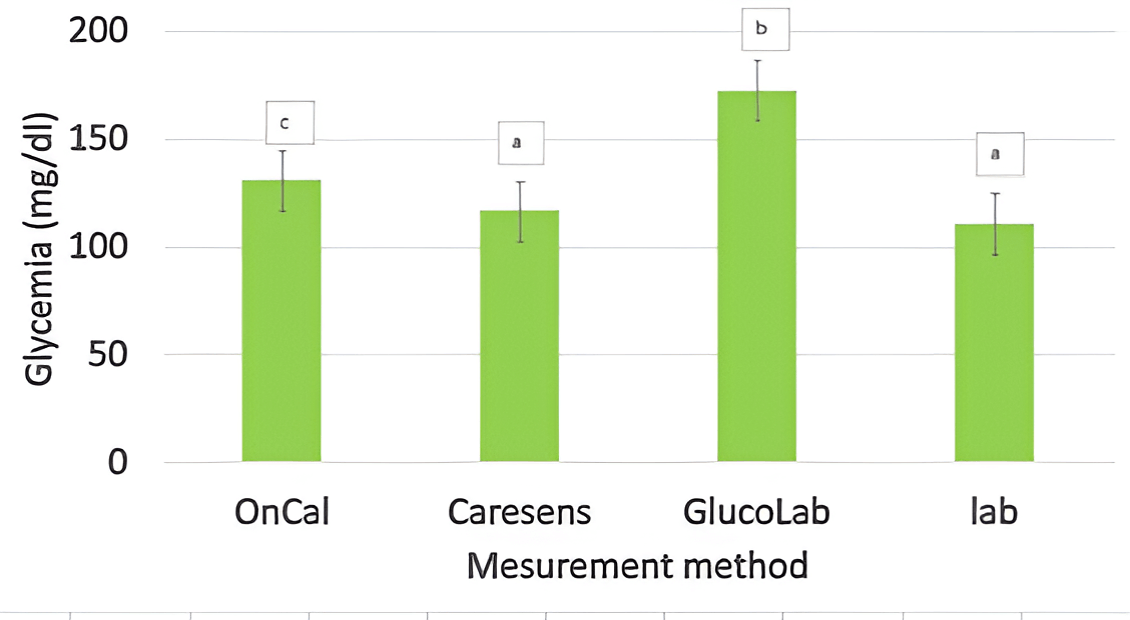
Figure 3: Glycemia measured by different glucometers or in the lab. Bars lacking a common letter differ (p<0.05). Error bars represent the standard error.
- Acknowledgements
Acknowledgments: the authors express their gratitude to ReachSci (University of Cambridge, UK) for this project start-up and encouraging. They extend their thanks to Elmaadani private analysis laboratory (Meknes, Morocco) for assisting in blood collection and handling.
- Conclusion
The glucometers used in Morocco are precise enough to be used to follow glycemia evolution. However, they should be better calibrated before sale for a better accuracy that allows the exact blood glucose determination. EDTA, mannitol, and urea, do not interfere with this determination.
References :
American Diabetes Association. Standards of Medical Care in Diabetes-2016 abridged for primary care providers. Clinical diabetes: a publication of the American Diabetes Association. 2016;34(1):3.
Bergenstal RM, rd JR. Global Consensus Conference on Glucose Monitoring Panel. The role of self-monitoring of blood glucose in the care of people with diabetes: report of a global consensus conference. Am J Med. 2005;118(9A):1S-6S.
Chan JCN, Lim L-L, Wareham NJ, Shaw JE, Orchard TJ, Zhang P, et al. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet 2020;396 (10267):2019–82. [PubMed: 33189186].
Czupryniak L, Barkai L, Bolgarska S, Bronisz A, Broz J, Cypryk K, et al. Self-monitoring of blood glucose in diabetes: from evidence to clinical reality in central and Eastern Europe recommendations from the international Central-Eastern European expert group. Diabetes Technology & Therapeutics. 2014;16(7):460-75.
Freckmann G, Baumstark A, Jendrike N, Zschornack E, Kocher S, Tshiananga J, et al. System accuracy evaluation of 27 blood glucose monitoring systems according to DIN EN ISO 15197. Diabetes Technology & Therapeutics. 2010;12(3):221-31.
Gomes T, Juurlink DN, Shah BR, Paterson JM, Mamdani MM. Blood glucose test strips: options to reduce usage. Canadian Medical Association Journal. 2010;182(1):35-38.
Heald AH, Stedman M, Davies M, Livingston M, Alshames R, Lunt M, et al. Estimating life years lost to diabetes: outcomes from analysis of National Diabetes Audit and Office of National Statistics data. Cardiovasc Endocrinol Metab. 2020;9(4):183–5.
Kalatehjary M, Sohrabi MB, Khosravi AA, Zolfaghari P. Correlation between blood glucose measured using glucometers and standard laboratory methods. Iranian Journal of Endocrinology and Metabolism. 2008;10(3):277-83.
Lagarde WH, Barrows FP, Davenport ML, Kang M, Guess HA, Calikoglu AS. Continuous subcutaneous glucose monitoring in children with type 1 diabetes mellitus: a single-blind, randomized, controlled trial. Pediatric Diabetes. 2006;7(3):159-64.
Magliano DJ, Islam RM, Barr ELM, Gregg EW, Pavkov ME, Harding JL, et al. Trends in incidence of total or type 2 diabetes: systematic review. BMJ 2019;366:l5003. [PubMed: 31511236].
R Core Team (2024). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. New England Journal of Medicine. 2001;345(19):1359-67.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual
author(s) and contributor(s) and not of MJHI and/or the editor(s). MJHI and/or the editor(s) disclaim responsibility for any injury to
people or property resulting from any ideas, methods, instructions or products referred to in the content.




